
May 16, 2025
| Today’s news and insights for biopharma leaders
|
NOTE FROM THE EDITOR
We’re less than three weeks away from our virtual event on cancer drug research! Along with our colleagues at PharmaVoice, we’ll be hosting executives from Merck & Co., Daiichi Sankyo and Akeso to unpack the latest in oncology.
Join us June 5 from 1 p.m. to 4:30 p.m. ET. You can register for free here.
|
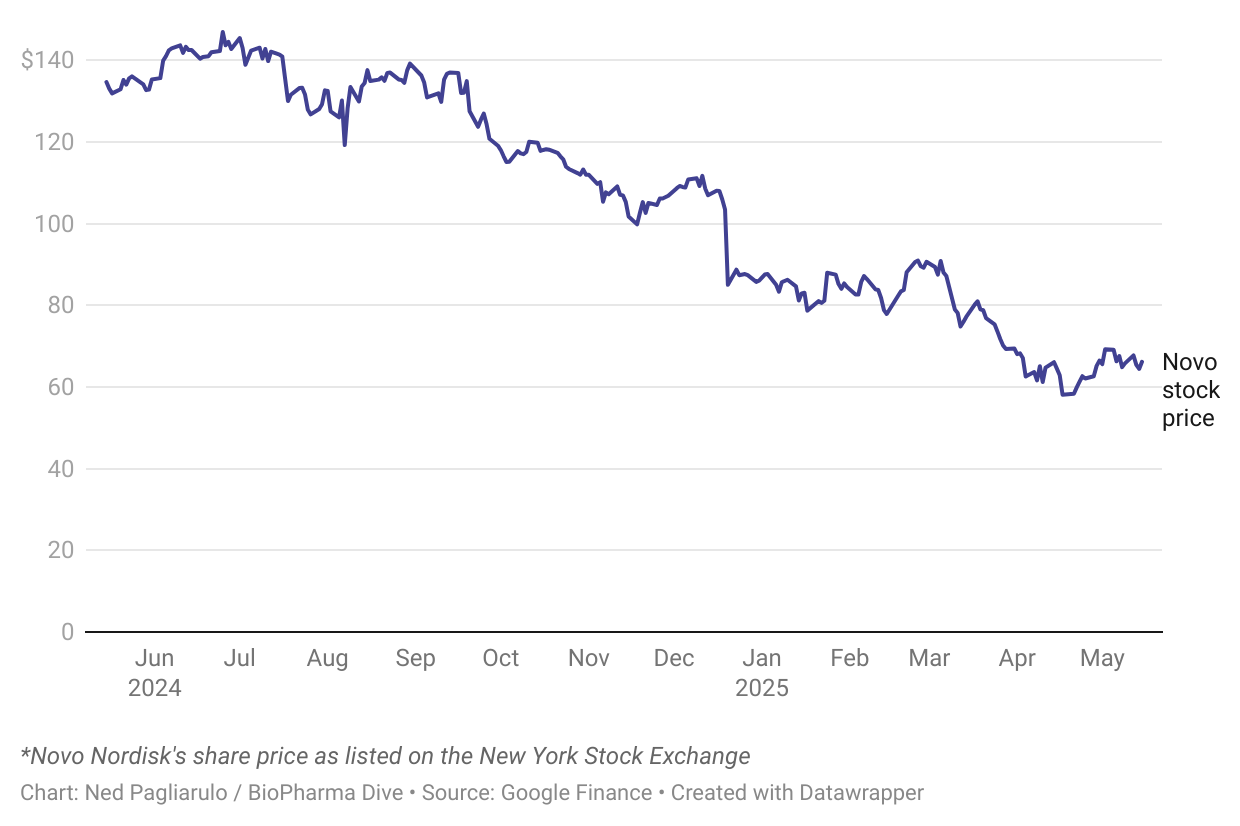
UPDATED
Lars Fruergaard Jørgensen will step down after eight years helming the Danish drugmaker, shares of which have shed half their value in the past 12 months.
|
A gene editing drug custom-made for a critically ill baby showed that, for some ultra-rare diseases, it’s possible to design and test a new CRISPR medicine in just a few months.
|
The deal “fits like a glove” for BioMarin’s business, according to an analyst who also thinks there could be a “valuable” market opportunity for Inozyme’s main drug.
|
Explore the cutting-edge of science and learn how Danaher is advancing healthcare innovation.
|
News roundup
A federal jury found Amgen liable for violating antitrust and tort laws. Elsewhere, Allogene and Kyverna trimmed staff and the FDA scheduled an advisory meeting.
|
UPDATED
Novartis and BioMarin Pharmaceutical, in striking recent deals to respectively buy biotechs Regulus and Inozyme, both made offers that valued their targets at triple-digit premiums.
|

|
Amidst the rise of biosimilars, market access challenges have become the definitive roadblock to bringing novel therapeutics to market. Learn how to build a market access plan in this webinar.
|
|
From Our Library
Playbook
Custom content for MRN
|
|